Which of the Following Statements About Redox Reactions Is False
State whether the following statements are true or false and correct the false statement. None of the above are true.

Solved Classify The Statements About Redox Reactions As True Chegg Com
OxygenH2O redox pair has the highest redox potential.

. The components of the electron transport chain are organized in terms of their redox potential. Redox reaction occurs simultaneously d. A Oxidation is the loss of electrons.
A secondary battery can operate either as a galvanic or electrolytic cell. -Redox reactions always involve charged species. Biochemistry Multiple Choice Questions on Biological Oxidation-Reduction Reactions.
-Electrons always move from an atom that attracts them less strongly. A reducing agent is one that undergo reduction. NADHNAD redox pair has the least redox potential.
NADHNAD redox pair has the least redox potential. State whether the given statement is true or false. B Reduction is the gain of electrons.
All of the above statement are true. An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species. For the reaction occurring in a voltaic galvanic cell G 0 c.
Electrolytic cells utilize electrical energy to drive non-spontaneous redox reactions b. Very Important Questions A cummulative deposit account of monthly instalment of 3600 at 9 pa. The reaction in the atomic reactor is a type of uncontrolled chain reaction.
A reducing agent gives electrons. Which of the following statements about redox reactions in biochemistry is FALSE. C A reaction can result in either oxidation or reduction not both.
Reduction is the process of gain of electrons by an atom ion or molecule. Which of the following statements about electrochemical cells is false. A A reaction involving elemental oxygen is a redox reaction.
C Reduction is the gain of electrons. N H 3. A if both sentences are trueB if both sentences are falseC if the first sentence is true but the second is false andD if the first sentence is false but the second is true.
E All of the above statement are true. Which of the following statements about redox potential is false. A O 2 is the strongest oxidant commonly encountered in biochemical processes.
-This type of reaction involves the transfer of electrons. OxygenH 2 O redox pair has the highest redox potential. Chemistry questions and answers.
1041 students attemted this question. A Oxidation is the loss of electrons. An acidic and a basic solution may be used to balance the redox reaction when the oxidation number method is.
Simple interest earns an interest of 17982. Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids. B Reduction is the gain of electrons.
Oxidation reaction occurs at the anode while reduction occurs at the cathode Question 6 Process of determining the nitrogen content of organic materials by mixing the sample with powdered copper II oxide and ignited to a combustion tube giving CO 2 H 2 O N 2 and small amounts of nitrogen oxides _____. D Oxidation is the loss of electrons. C FAD is a stronger oxidizing agent than NAD.
E All of the above statement are true. Reduction is the gain of electrons. 250 TOP MCQs on Biological Oxidation-Reduction Reactions and Answers.
10 Which of the following statements about redox reactions is FALSE. B Most biological oxidations involve reactions between an organic substrate and O 2. C A reaction can result in either oxidation or reduction not both.
B A reaction can result in either oxidation or reduction not both. D A reaction involving elemental oxygen is a redox reaction. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron.
A g B r is soluble in a q. E All of the above statement are true. Which of the following statements about redox potential is false.
Which of the following statements about redox reactions is FALSE. D A reaction involving elemental oxygen is a redox reaction. Oxidation is the loss of electrons.
Biochemistry Biological Oxidation Reduction Reactions. For the given aqueous reactions which of the following statements isare true. Which of the following statements about redox reactions is false.
Oxidation is the process of loss of electrons by a chemical species atom ion or molecule. SCIA REDOX reaction can result in either oxidation or reduction not both D. Which of the following statements regarding redox reactions is incorrect.
Moving to another question will save this response.
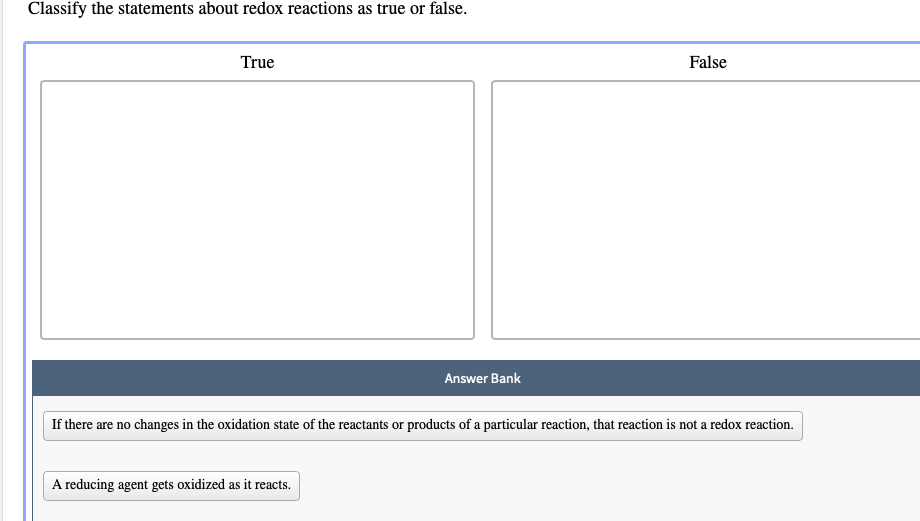
Solved Classify The Statements About Redox Reactions As True Chegg Com
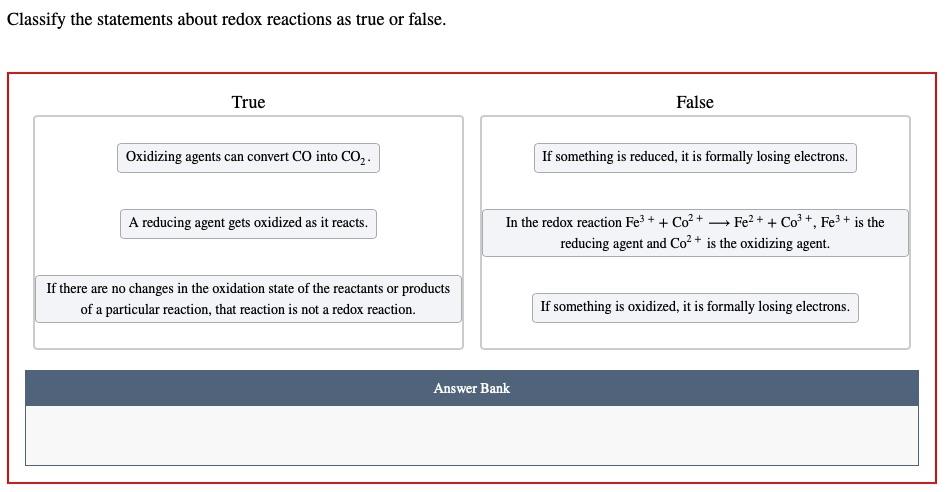
Solved Classify The Statements About Redox Reactions As True Chegg Com
Belum ada Komentar untuk "Which of the Following Statements About Redox Reactions Is False"
Posting Komentar